Identifying Women Who Can Undergo Observation of High-Risk Breast Lesions Instead of Surgical Excision
Literature review notes a multidisciplinary approach, including preventative therapies, can guide decision making

In concert with the RadioGraphics monograph, RSNA News shares the latest in breast cancer research, intervention and patient education.
High-risk lesions of the breast are frequently encountered in percutaneous biopsy specimens. These lesions are common and found in 2% to 14% of core biopsy specimens. Flat epithelial hyperplasia (FEA) and atypical ductal hyperplasia (ADH) are two of the most common, which have been found to be potentially overtreated.
FEA and ADH both exhibit atypia of ductal epithelial cells, however FEA maintains a normal ‘flat’ luminal surface while ADH exhibits architectural changes including bridges and micropapillary projections. While these are not considered to be breast cancer, they have the risk of being upgraded to malignancy at complete surgical excision or developing into malignancy over time. Thus, they are termed high-risk lesions of the breast.
“Each patient diagnosed with a high-risk lesion, including FEA and ADH, should have a tailored discussion of management considerations individualized to their case. The discussion should include weighing the benefits of early detection and treatment of upgraded lesions against overdiagnosis and overtreatment of lesions that are ultimately not upgraded,” said Laura K. Harper, MD, assistant professor of radiology, Division of Breast Imaging, Mayo Clinic, Phoenix. “Patients are ideally fully informed and can then participate in shared decision making for surgical excision versus observation. While many patients do go on to opt for excision after hearing ‘breast cancer,’ those that are good candidates for observation may feel more comfortable choosing active surveillance.”
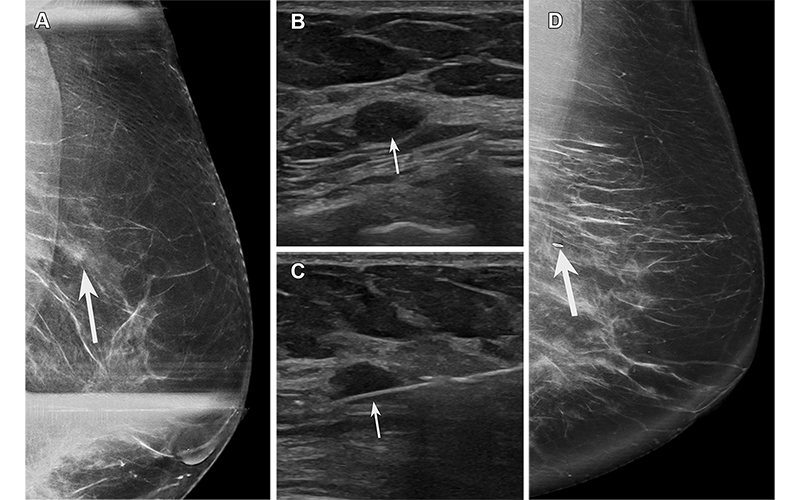
FEA found at biopsy of a hypoechoic mass in a 64-year-old asymptomatic woman recalled from screening mammography. (A) Spot compression exaggerated craniocaudal lateral (XCCL) mammogram of the left breast shows an asymmetry (arrow) in the outer breast at a posterior depth. (B) US image of the left breast shows a corresponding irregular hypoechoic mass (arrow). (C) Image from US-guided biopsy shows a needle (arrow) traversing the mass. (D) Postprocedure XCCL mammogram of the left breast shows a marking clip (arrow) in the expected position. Pathologic analysis yielded FEA on a background of proliferative fibrocystic changes, considered elevated risk and possibly discordant due to the presence of a mass. The patient went on to surgical excision, which yielded FEA and fibroadenoma. The fibroadenoma accounts for the mass at imaging and was likely undersampled at core biopsy. https://pubs.rsna.org/doi/10.1148/rg.230016 © RSNA 2023
FEA Includes Mild Cytologic Atypia
The distinguishing features of FEA are the replacement of the single layer of native epithelial cells by a single or three to five layers of monotonous atypical cuboidal to columnar cells demonstrating nuclear enlargement, higher nuclear-cytoplasmic ratio and hyperchromatic nuclei without evidence of intraluminal proliferation of the cells.
“Originally described as ‘clinging carcinoma in situ,’ the updated term ‘flat’ refers to the lack of significant intraepithelial proliferation and architectural atypia,” Dr. Harper said. “Given the lack of architectural alteration, FEA may be easily overlooked on histopathologic examination and there is wide variation in the diagnosis of FEA among pathologists.”
FEA is increasingly being diagnosed at core biopsy due to its association with calcifications detected mammographically. The term “pure” FEA is used when FEA is the most advanced lesion visualized at histopathology and is encountered in 1% to 2% of benign breast biopsies.
The most common imaging presentation of pure FEA is calcifications seen on mammography. Calcifications are typically amorphous and grouped, although they may present in varying morphology and distribution. There is very little literature on the MRI imaging findings associated with FEA diagnosed at biopsy
The imaging presentation of FEA has been shown to influence the upgrade rate to malignancy. In previous studies, the upgrade rate was higher for MRI-only detected lesions (27%) compared to mammography-only lesions (9%), ultrasound-only lesions (14%) and lesions seen by both mammogram and ultrasound (9%).
Patient factors have also been shown to affect the upgrade rate of FEA. The upgrade rate has been shown to be higher when the patient has a personal history of breast cancer and/or a concurrent cancer diagnosis on a separate core biopsy.
ADH Can Mimic Cellular Changes of Ductal Carcinoma In Situ
ADH has both cytologic and architectural atypia involving a restricted number of luminal epithelial cells. These cellular changes of ADH are identical to ductal carcinoma in situ (DCIS), with diagnostic distinction based on size only.
“Frequent interobserver variation has been demonstrated between pathologists in distinguishing ADH from DCIS on biopsy specimens,” Dr. Harper said. “Given the uncertainty with distinguishing between ADH and DCIS on limited core needle biopsy samples, some researchers propose the term ‘atypical intraductal epithelial proliferation’ rather than ADH with a final diagnosis based on excision.”
ADH is often diagnosed by core needle biopsy, but Dr. Harper cautions that it should be considered that such proliferations may represent the periphery of a more established lesion of DCIS. However, ADH alone is considered a nonobligate precursor to malignancy.
Calcifications on mammography are the most common imaging presentation of ADH and typically appear amorphous and grouped.
The most common sonographic appearance associated with the upgrade rate of ADH after needle biopsy was that of an irregular microlobulated mass with mild hypoechogenicity and no acoustic enhancement or shadowing.
The reported upgrade rates for ADH diagnosed on percutaneous biopsy are widely variable in the literature, ranging from 0%–84%. Similar to FEA, this wide range is due to a large number of single-center, retrospective studies with limited number of cases and variations in study design and long-term outcome follow-up.
“There are several factors that affect the ADH upgrade rate to malignancy. Patient factors that increase upgrade rate include a personal history of breast cancer, age less than 50 years old, and dense breast tissue. On imaging, calcifications that demonstrate a suspicious morphology, are more extensive in size (>10mm) and are associated with a mass increase the likelihood of upgrade to malignancy,” Dr. Harper said. “Following biopsy, histopathologic specimens that show a micropapillary pattern, necrosis and involve >2-3 foci have a higher upgrade rate. The radiologist should carefully consider these patient, imaging and pathology factors when assessing radiology-pathology concordance and issuing recommendations.”
Patient Management Is Key
Emerging evidence suggests that a uniform approach of excision may not be appropriate for all high-risk breast lesions, and that with adequate sampling and a benign, concordant core biopsy result some lesions may be safely left in situ with appropriate clinical and imaging surveillance.
This shift toward a more tailored multidisciplinary approach to conservative management of high-risk lesions may be considered to reduce patient anxiety, overtreatment and health care costs in cases where upgrade rates are determined to be low enough to obviate surgery.
“The rate at which ADH will be upgraded to breast cancer is variable, estimated to be up to 29% by two large meta-analyses. Additionally, a diagnosis of ADH confers a fourfold increased risk for the development of future cancer in both breasts,” Dr. Harper concluded. “All patients should be counseled on risk reduction strategies, including pharmacologic risk reduction with selective estrogen-receptor modulators and aromatase inhibitors and lifestyle modifications, such as maintaining a normal body mass index and limiting alcohol intake.”
For More Information
Access the RadioGraphics paper, "Imaging Characteristics of and Multidisciplinary Management Considerations for Atypical Ductal Hyperplasia and Flat Epithelial Atypia: Review of Current Literature."
Read previous RSNA News articles on breast imaging:
- Radiologists Play a Key Role in Assessing Radial Scars
- Understanding How Pregnancy and Breastfeeding Impact Breast Imaging and Diagnosis
- Enhancing the Evaluation of Breast Symptoms in the ED
- Closing the Breast Cancer Screening Gap for Uninsured Patients
- Radiologists Should Ready Their Practices For Automated US Breast Screening