Guidelines Will Boost Interventional Oncology Research
Guidelines will provide the foundation for scientific reproducibility between interventional oncology studies

The development of new research guidelines for interventional oncology that standardize treatment outcomes and the reporting of data represents a major step forward for an increasingly important medical subspecialty, according to a report in Radiology.
Treatment effectiveness is measured through a variety of means such as disease-free and progression-free survival. A lack of consensus regarding these parameters in oncology-related studies and how to uniformly share these outcomes amongst investigators worldwide has created a host of problems. Issues like different complication-related outcomes reported for the same treatment modality and inconsistencies in the reporting of overall survival data means that study results cannot always be reliably compared.
“Study results are being collected, analyzed and reported in many different ways, and we tend not to speak the same language within the field of clinical oncology,” said study lead author Robbert S. Puijk, MD, a radiology resident and researcher at the Onze Lieve Vrouwe Gasthuis Hospital and Amsterdam University Medical Centers in Amsterdam.
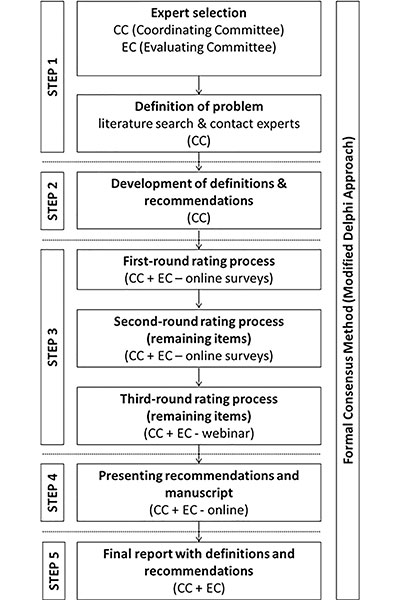
To address these shortcomings, an international panel of 62 experts recently convened and developed important recommendations on how to uniformly collect, analyze and report outcomes for patients treated with image-guided tumor ablation.
New Guidelines Will Ensure Objective and Reliable Study Outcomes
Among key recommendations, the panel determined that, to compare different treatment techniques, outcomes should be analyzed and reported per patient and per tumor. This is because multiple index tumors within one unique patient, such as multiple liver metastases from colorectal cancer, are often treated simultaneously and cannot be regarded as independent.
The panel agreed that parameters like overall survival and disease-free survival should be analyzed per patient and not on a per tumor or per procedure basis. Parameters that address both procedure-related side-effects and direct costs such as short-term complications and anesthesia techniques should be addressed per procedure, the panelists concluded.
The panelists reached several other important agreements, including ones regarding the definitions for recurrence-free, disease-free and progression-free survival.
The new guidelines will boost interventional oncology in a variety of ways, according to Dr. Puijk.
“The given definitions in these current guidelines will provide the necessary foundation for scientific reproducibility between interventional oncology studies as they will ensure an objective and reliable interpretation of study outcomes, allow for accurate comparisons of results and avoid misinterpretations,” he said.
Dr. Puijk called the participation of a large number of international experts on the panel a key motivating factor to putting the guidelines on paper.
“Together with the help of independent biostatisticians and epidemiologists, it strengthens our methodology and indicates the importance of this project,” he said. “Widespread adoption of these guidelines is another step forward in the professionalization of our field, interventional oncology.”
The researchers hope to extend these guidelines to regional and systemic cancer treatments and ultimately attract the participation of other medical societies.
“With this interchangeable setup and a similar study design we intend to follow up this project regularly,” Dr. Puijk said.
The project represented a collaboration between the Society of Interventional Oncology (SIO) and the Definition for the Assessment of Time-to-event Endpoints in Cancer (DATECAN) trials initiative. Dr. Puijk’s colleague Martijn R. Meijerink, MD, PhD, was senior author on the paper.
For More Information
Access the Radiology paper, “Consensus Guidelines for the Definition of Time-to-event End Points in Image-guided Tumor Ablation: Results of the SIO and DATECAN Initiative.”
Read previous RSNA News stories on interventional oncology:
Visit the RSNA Online Learning Center for CME-activities related to interventional oncology.