Research On New MRI Contrast Agent Wins 2022 Chynn Award
International research showed clinically significant increase in efficacy

A study on a promising new MRI contrast agent that uses a lower concentration of gadolinium than existing agents, but may deliver equivalent or better diagnostic results, has won the 2022 Kuo York Chynn Neuroradiology Research Award.
Gadopiclenol, a gadolinium-based contrast agent (GBCA) recently approved by the FDA and currently under review by the European Medicines Agency (EMA), was studied in a Phase III trial to determine its safety and efficacy compared to a more commonly used GBCA.
Gadopiclenol was configured to permit gadolinium atoms to interact with twice as much water as existing agents. This increases relaxivity and tissue enhancement, enabling lower doses that could reduce any potential risks associated with gadolinium exposure.
“Ultimately, this could mean that radiologists will have a contrast agent that enables them to give less gadolinium and yet get better results in terms of diagnosis than with the currently existing agents,” said Leo J. Wolansky, MD, professor and chair of radiology at the University of Connecticut School of Medicine in Farmington, CT, and this year’s Kuo York Chynn Neuroradiology Research Award recipient.
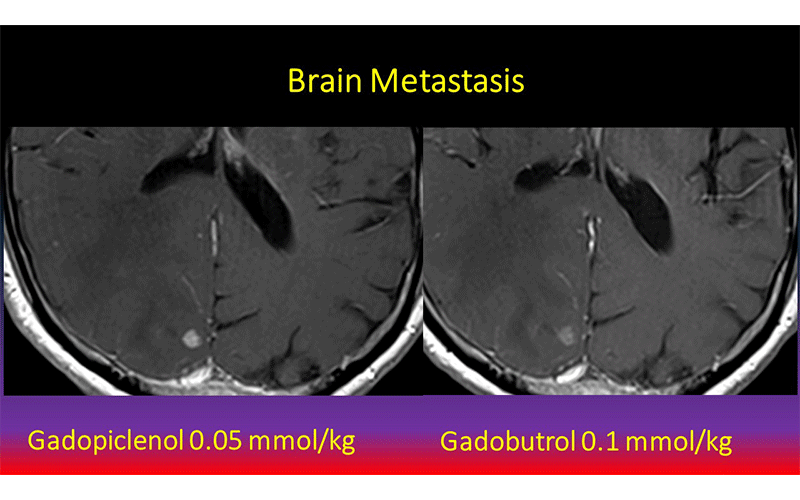
Contrast enhanced MRI with Gadopiclenol (left) better demonstrating a brain metastasis than comparator agent (right). Images courtesy of J. Hao.
Study Compared New Agent With Commonly Used One
In the PICTURE Trial, (gadoPIClenol for cenTral nervoUs system magnetic REsonance),
Dr. Wolansky and colleagues compared gadopiclenol to gadobutrol, a commonly used GBCA, for contrast-enhanced MRI of the central nervous system (CNS). The gadopiclenol was administered at a dose of 0.05 mmol/kg and the gadobutrol was given at a dose of 0.1 mmol/kg.
The study group, drawn from 33 centers in 11 countries, included 256 patients with CNS lesions who were randomized in a double-blinded manner to undergo two MRIs, with gadopiclenol then gadobutrol or vice versa. Three independent off-site readers who were blinded to the agent used assessed the images based on three parameters: border delineation, internal morphology and contrast enhancement. Three other independent readers conducted objective measurements and compared the scans with the two agents for their overall diagnostic preference, also in blinded fashion.
The degree of enhancement and lesion-to-background ratio were rated superior for gadopiclenol by all readers. Contrast-to-noise ratio was rated superior for gadopiclenol for two readers. Overall diagnostic preference was significantly higher for gadopiclenol (p<0.001).
“Even when given at half the dose of a currently available agent, the gadopiclenol was mildly more effective at showing lesions in the brain,” Dr. Wolansky said.
New GBCA Will Continue To Be Investigated
GBCAs have been used for decades and have an excellent safety profile. However, in patients with kidney failure, concerns exist regarding a reported association between GBCA exposure and development of nephrogenic systemic fibrosis, a potentially fatal disease.
More recently, researchers have found trace gadolinium deposits retained in the brains of some patients who have received GBCAs, although the implications of these deposits are unknown. In response, the U.S. Food and Drug Administration (FDA) issued a black box warning advising users to consider the benefits and risks in every patient. Furthermore, the EMA has recommended that the lowest dose possible of gadolinium be used providing that clinical efficacy is not compromised. The “non-inferiority” of gadopiclenol at half the dose of gadolinium appears to satisfy this recommendation.
Gadopiclenol received FDA approval in September 2022 and is expected to be available in the summer of 2023. Phase IV trials are planned and will assess the agent in different settings, including at full and three-quarter strength dosages.
“In this study, we showed a mild but clinically significant increase in performance for Gadopiclenol,” Dr. Wolansky said. “Future studies could show that the three-quarter dose would be much more effective than any currently available agent.”
Dr. Wolansky, who presented the research at RSNA 2022, credited the study’s success to his collaborators and researchers in the previous trials.
“This research is the culmination of many years of research by many people,” he said. “I inherited all the great work that was done prior to my involvement.”
Funded with a donation by internationally renowned neuroradiologist and longtime RSNA member Kuo York Chynn, MD, the RSNA Kuo York Chynn Neuroradiology Research Award provides $3,000 to the author of the top neuroradiology research paper presented at the RSNA annual meeting.
“It’s a huge honor,” Dr. Wolansky concluded. “I’m so grateful that the Chynn family created this award and that, among the numerous abstracts the RSNA received, they selected mine.”
For More Information
Access the RSNA 2022 session, “Efficacy and Safety of Gadopiclenol for Central Nervous System (CNS) Magnetic Resonance Imaging (MRI): The PICTURE Trial,” at Meeting.RSNA.org.
Read RSNA News stories on previous Chynn Award recipients: