AI Predicts Lung Cancer Risk
Distinguishing between benign and malignant nodules is crucial to catch cancers early


An artificial intelligence (AI) program accurately predicts the risk that lung nodules detected on screening CT will become cancerous, according to a study in Radiology.
Lung cancer is the leading cause of cancer death worldwide, with an estimated 1.8 million deaths in 2020, according to the World Health Organization. Low-dose chest CT is used to screen people at a high risk of lung cancer, such as longtime smokers. It has been shown to significantly reduce lung cancer mortality, primarily by helping to detect cancers at an early stage when they are easier to treat successfully.
While lung cancer typically shows up as pulmonary nodules on CT images, most nodules are benign and do not require further clinical workup. Accurately distinguishing between benign and malignant nodules is therefore crucial to catch cancers early.
Algorithm Can Aid in Estimating Malignancy Risk
For the new study, researchers developed an algorithm for lung nodule assessment using deep learning. The researchers trained the algorithm on CT images of more than 16,000 nodules, including 1,249 malignancies, from the National Lung Screening Trial. They validated the algorithm on three large sets of imaging data of nodules from the Danish Lung Cancer Screening Trial.
The deep learning algorithm delivered excellent results, outperforming the established Pan-Canadian Early Detection of Lung Cancer model for lung nodule malignancy risk estimation. It performed comparably to 11 clinicians, including four thoracic radiologists, five radiology residents and two pulmonologists.
“The algorithm may aid radiologists in accurately estimating the malignancy risk of pulmonary nodules,” said the study’s first author, Kiran Vaidhya Venkadesh, a PhD candidate with the Diagnostic Image Analysis Group at Radboud University Medical Center in Nijmegen, the Netherlands. “This may help in optimizing follow-up recommendations for lung cancer screening participants.”
The algorithm potentially brings several additional benefits to the clinic, the researchers said.
“As it does not require manual interpretation of nodule imaging characteristics, the proposed algorithm may reduce the substantial interobserver variability in CT interpretation,” said senior author Colin Jacobs, PhD, assistant professor in the Department of Medical Imaging at Radboud University Medical Center in Nijmegen. “This may lead to fewer unnecessary diagnostic interventions, lower radiologists’ workload and reduce costs of lung cancer screening.”
The researchers plan to continue improving the algorithm by incorporating clinical parameters like age, sex and smoking history.
They are also working on a deep learning algorithm that takes multiple CT examinations as input. The current algorithm is highly suitable for analyzing nodules at the initial, or baseline, screening, but for nodules detected at subsequent screenings, growth and appearance in comparison to the previous CT are important.
Dr. Jacobs and colleagues have developed other algorithms to reliably extract imaging features from the chest CT related to chronic obstructive pulmonary diseases and cardiovascular diseases. They will be investigating how to effectively integrate these imaging features into the current algorithm.
For More Information
Access the Radiology study, “Deep Learning for Malignancy Risk Estimation of Pulmonary Nodules Detected at Low-Dose Screening CT.”
Read previous RSNA News articles on lung cancer screening:
- Lung Cancer Screening Makes Gains As A Detection Tool
- Researchers Examine Barriers to Low-Dose CT Lung Cancer Screening
- Experts Issue Guide on Lung Cancer Screening, Management During COVID-19
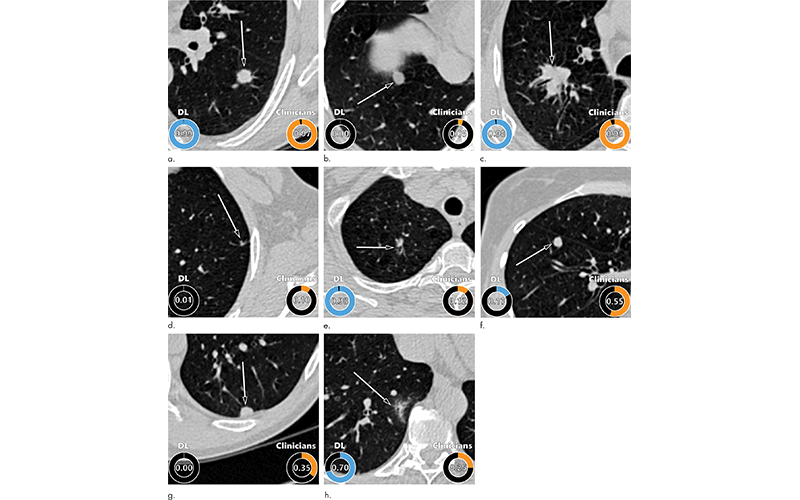
Examples of CT images in nodules from the Danish Lung Cancer Screening Trial (DLCST) with (a–d) high and (e–h) low agreement between the deep learning (DL) algorithm and the clinicians for malignancy risk estimation. Numbers in rings on bottom left of each image are the algorithm’s malignancy score, and numbers in rings on bottom right of each image are the clinicians’ median malignancy score. The extent of the color filling is proportional to the malignancy risk (on a scale of 0 to 1, where 0 represents the lowest risk and 1 represents the highest risk). (a) Image shows a 15-mm spiculated and lobulated malignant nodule (arrow) classified correctly by the DL algorithm and clinicians. (b) Image shows an 11-mm smooth benign nodule (arrow) classified correctly by the DL algorithm and clinicians. (c) Image shows a 29-mm benign lesion (arrow) suspected to be a malignant nodule by both the DL algorithm and clinicians. This participant was diagnosed with pneumonia at clinical workup. (d) Image shows a 5-mm malignant nodule (arrow) called benign by both the DL algorithm and clinicians. The growth of the nodule can be seen from follow-up CT examinations. (e) Image shows a 15-mm part-solid malignant nodule (arrow) classified correctly by the DL algorithm and not suspected to be malignant by seven of 11 clinicians. (f) Image shows an 8-mm benign nodule (arrow) predicted to be moderately suspicious by the clinicians and called benign by the DL algorithm. (g) Image shows an 11-mm malignant nodule (arrow) predicted to be moderately suspicious by most clinicians but called benign by the DL algorithm. (h) Image shows a 16-mm benign lesion (arrow) classified correctly by the clinicians and predicted to be highly suspicious by the DL algorithm.