Brain Iron Accumulation Linked to Cognitive Decline in Alzheimer’s Patients
MRI-based iron mapping could be a useful tool for treatment monitoring
Researchers using MRI have found that iron accumulation in the outer layer of the brain is associated with cognitive deterioration in people with Alzheimer’s disease (AD), according to a study published in the journal Radiology.
Previous research has linked abnormally high levels of iron in the brain with AD. Iron deposition correlates with amyloid beta, a protein that clumps together in the brains of people with AD to form plaques that collect between neurons and disrupt cell function. Associations have also been found between iron and neurofibrillary tangles, abnormal accumulations of the protein tau that collect inside neurons. These tangles block the neuron’s transport system, which harms the communication between neurons.
It is known that deep gray matter structures of patients with AD contain higher brain iron concentrations. Less is known about the neocortex, the deeply grooved outer layer of the brain that is involved with language, conscious thought and other important functions. The neocortex is challenging to assess by MRI, as the anatomy of the area makes MRI prone to distortions, signal decays and artifacts.
“The best solution to minimize these artifacts would be using ultra-high-resolution scans,” said study co-author Reinhold Schmidt, MD, professor of neurology and chairman of the Department of Neurology at the Medical University of Graz in Graz, Austria. “However, in the clinical setting, scan time is a limiting factor, and a compromise has to be found.”
Results Support Using Chelators to Remove Excess Iron from Body
For the new study, Dr. Schmidt and colleagues developed an approach using a 3T MRI scanner that allowed the best tradeoff between resolution and scan time, along with postprocessing to correct the influence of the distortions.
They used MRI to investigate baseline levels of brain iron in 100 individuals with AD and 100 healthy controls. Of the 100 participants with AD, 56 had subsequent neuropsychological testing and brain MRI at a mean follow-up of 17 months.
The technique enabled the researchers to create a map of brain iron, determining iron levels in parts of the brain like the temporal lobes, or the areas of the brain lying underneath the temples, and the occipital lobes in the back of the head.
“We found indications of higher iron deposition in the deep gray matter and total neocortex, and regionally in temporal and occipital lobes, in Alzheimer’s disease patients compared with age-matched healthy individuals,” Dr. Schmidt said.
The brain iron accumulation was associated with cognitive deterioration independently of brain volume loss. Changes in iron levels over time in the temporal lobes correlated with cognitive decline in individuals with AD.
“These results are all in keeping with the view that high concentrations of iron significantly promote amyloid beta deposition and neurotoxicity in Alzheimer’s disease,” Dr. Schmidt said.
The results point to a potential role in AD treatment for drugs that reduce the iron burden in the brain. These drugs, known as chelators, can remove excess iron from the body.
“Our study provides support for the hypothesis of impaired iron homeostasis in Alzheimer’s disease and indicates that the use of iron chelators in clinical trials might be a promising treatment target,” Dr. Schmidt said. “MRI-based iron mapping could be used as a biomarker for Alzheimer’s disease prediction and as a tool to monitor treatment response in therapeutic studies.”
For More Information
Access the Radiology study, “Cross-sectional and Longitudinal Assessment of Brain Iron Level in Alzheimer Disease using 3-T MRI."
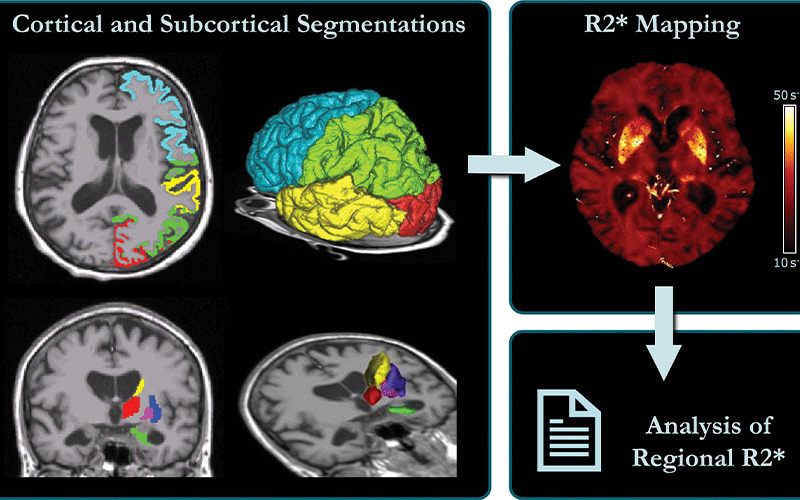
Schematic representation of MRI processing. Left: FreeSurfer-derived cortical and subcortical segmentations overlaid on a T1-weighted scan were affinely registered into R2∗ space. Right: R2∗ map corrected for macroscopic field inhomogeneities, where median R2∗ values were calculated for each region and then used for further statistical analyses.