Theranostics Advances Precision Medicine for Cancer Patients
Pairing diagnostic biomarkers with therapeutic agents to target diseased cells


Known as the father of theranostics, Saul Hertz, MD, founding director of the Thyroid Unit at Massachusetts General Hospital in Boston, pioneered the first targeted cancer therapies. In 1941, he administered radioiodine to a patient with Graves’ disease, a type of hyperthyroidism. The research published following the treatment marked a seminal move forward for nuclear medicine and marked the beginning of theranostics—personalized cancer treatment that, as the name suggests, combines therapeutics and diagnostics imaging.
More than 80 years after Dr. Hertz’s initial work, the field is booming thanks to recent advances in molecular biology. Theranostic agents are used today to treat prostate cancer and neuroendocrine tumors, and they are under investigation for the diagnosis and treatment of numerous other cancers.
“The clinical use of theranostics has grown dramatically in recent years,” said Brian J. Burkett, MD, MPH, a radiologist subspecialized in nuclear medicine and neuroradiology at Mayo Clinic in Rochester, MN. “It offers a health care team a clear picture of a cancer's nature and scope in the body and enables more selective treatment based on a molecular target while reducing the risk of damage to normal tissues.”
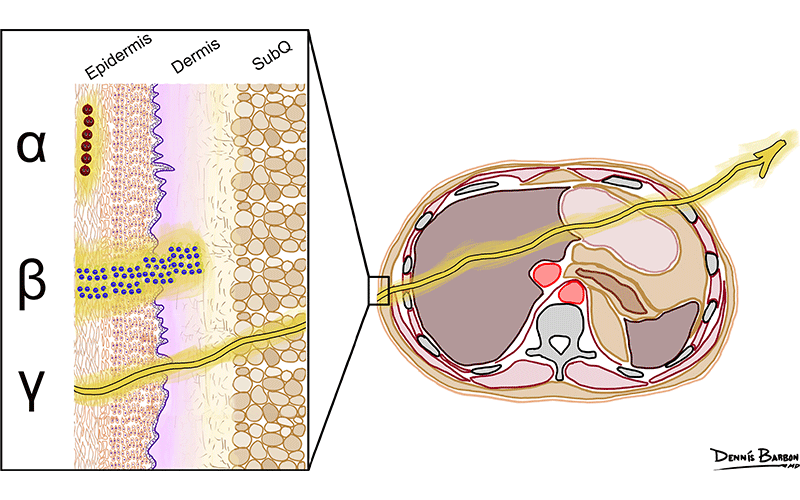
Relative ranges of different types of radiation in tissue. Note that β radiation has a longer range than α radiation in tissue; however, both particles remain relatively localized, causing tissue damage. In contrast, γ radiation has a much longer range; it leaves the body and therefore is useful for imaging. SubQ = subcutaneous. https://doi.org/10.1148/rg.230097 ©RSNA 2023
Combining Imaging and Molecular Radiotherapy
Theranostic agents contain a biologic component that binds to a specific molecular target in a patient’s tumor, making them ideally suited for today’s push toward precision medicine.
“The target might be different for different cancers,” said Nadine Mallak, MD, associate professor of body imaging and nuclear medicine at Oregon Health and Science University in Portland. “It could be a ligand, an antigen, or a receptor. It has to be somewhat specific to the given tumor so that the tumor can accept it relative to normal organs.”
The biologic component of a theranostic agent can be labeled with either positron emitters for PET-based imaging or beta and alpha emitters for targeted radionuclide therapy.
A prominent recent example is 177-Lu DOTATATE, an agent where a radioisotope of the element lutetium is linked to a biologic molecule that targets the peptide receptors, such as somatostatin, overexpressed in neuroendocrine tumors. In 2018, the U.S. Food and Drug Administration (FDA) approved 177-Lu DOTATATE for the treatment of metastatic neuroendocrine tumors of the pancreas and midgut. Four years later, 177-Lu-PSMA-617, an agent that targets prostate-specific membrane antigen (PSMA), won FDA approval for the treatment of metastatic castrate-resistant prostate cancer.
“These therapies are now frequently used in my practice at the Mayo Clinic,” Dr. Burkett said. “The increased volume has fostered the development of a busy dedicated nuclear medicine therapy center, staffed by a nuclear medicine physician and team of nurses, technologists, and advanced practice providers each day.”
Sequencing of Theranostics Relative to Other Treatments
Theranostic agents provide another systemic treatment option for cancer patients who may have already undergone chemotherapy and/or immunotherapy. Recent evidence suggests that they may be more effective when used earlier, for example, before chemotherapy for prostate cancer patients.
Compared to external beam radiation therapy (EBRT) which delivers local radiation to certain tumors, theranostic agents are systemic treatments that treat tumors expressing the target throughout the body. Since theranostic agents are specifically targeted to a feature of the tumor cells, they may cause less damage to healthy tissues surrounding the tumor relative to EBRT. Due to their systemic circulation, however, they have a different safety profile with side effects such as bone marrow toxicity.
Compared to chemotherapy, radionuclide therapy is a more selective and personalized approach that is usually more tolerable with fewer side effects. For example, Lu-177-PSMA-617 for prostate cancer has been shown to cause fewer adverse effects than standard-of-care chemotherapy for metastatic prostate cancer.
“Many of my patients report that Lu-177-PSMA-617 was less difficult to tolerate than chemotherapy,” said Dr. Burkett, the lead author of a 2023 review of theranostics that appeared in Radiology: Imaging Cancer. “Furthermore, with a theranostic approach, we can use a diagnostic scan prior to therapy to see where the therapeutic drug will localize and confirm likelihood of response to radionuclide therapy.”
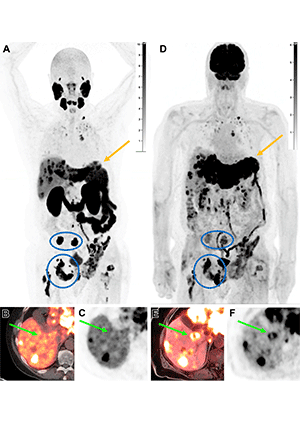
Intraindividual tumor heterogeneity in a 78-year-old man with progressing metastatic castration-resistant prostate cancer previously treated with hormonal therapy and docetaxel, who was referred for consideration of 177Lu–PSMA-617 therapy. 18F-DCFPyL PET/CT was performed to assess eligibility for treatment. (A–C) Coronal MIP (A), axial PET/CT (B), and axial PET (C) images show increased PSMA uptake in metastatic disease to the bones, liver, lungs, and supraclavicular nodes. However, several liver lesions are photopenic (arrow in B and C), concerning for PSMA-negative disease, which prompted evaluation with 18F–fluorodeoxyglucose (FDG) PET. (D–F) Coronal MIP (D), axial PET/MRI (E), and axial PET (F) images from 18F-FDG PET/MRI performed 1 month later show FDG uptake in the PSMA-negative liver lesions (arrow in E and F), in keeping with discordant FDG-avid PSMA-negative disease, concerning for more aggressive disease. Note the enlargement of the left hepatic lobe from quick progression of hypermetabolic metastases (arrow in A and D). The bone metastases show more intense uptake with PSMA than with FDG (blue circle and oval in A and D). On the basis of these findings, the patient was not considered a suitable candidate for RPT and was considered for second-line chemotherapy instead. https://pubs.rsna.org/doi/10.1148/rg.230097 ©RSNA 2023
Dosimetry Is Key to Future Uses
Experts agree that improvements in radiation dosimetry will be key to optimizing theranostics in the future.
Compared with EBRT, the radiation dose for theranostics is lower but is delivered over a longer period. This allows for tissue and DNA repair, which means that normal organs will tolerate higher doses than they would with EBRT.
However, the limits are starting to come into focus as more data comes in. Dr Mallak, senior author of a guide to theranostics for physicians and medical physicists that appeared in RadioGraphics, noted that dose limits to the kidneys with EBRT is 23 Gray (GY); doses higher than that will harm the organ. With theranostics, there is evidence that radiation doses to the kidney can go up to more than 30 GY.
“This means we can achieve higher activities of the therapeutic agent before reaching the toxicity to the kidneys,” Dr. Mallak said. “So, we know we can go with higher doses with theranostics, but the dose limits to normal organs need to be established.”
While dosimetry is routinely used in planning EBRT and brachytherapy, it’s more complex for radionuclide therapies due in part to patient-specific variation in the distribution of tumors, variable biodistribution and kinetics of the radiopharmaceutical, and challenges in consistent quantification of radiation from SPECT systems.
Until more is known about dose limits, theranostics is being performed conservatively.
“These agents work really well and they’re already helping a lot of patients,” Dr. Mallak said. “Once we have more dosimetry data, we can take it even further to personalize treatment for our patients.”
Future and Potential Limitations
Along with improvements in dosimetry optimizations, the implementation of newer radiopharmaceuticals is expected to boost theranostic treatments. Targeted alpha-particle therapies show promise, as they deliver high energy to target sites within a short range and therefore inflict more damage to the tumor and expose adjacent normal tissues to less radiation.
Researchers are also exploring the use of theranostics in novel combinations with other treatment modalities like chemotherapy and immunotherapy.
“All of these developments have potential to augment the use of theranostics in cancer treatment in the future,” Dr. Burkett concluded.
Researchers have acknowledged that there are limitations with theranostics, including that not many patients are candidates and, those that are, risk the potential for bone marrow toxicity or that other organs may be affected by the treatment.
For More Information
Access the Radiology: Imaging Cancer article, “A Review of Theranostics: Perspectives on Emerging Approaches and Clinical Advancements.”
Access the RadioGraphics article, “Essentials of Theranostics: A Guide for Physicians and Medical Physicists.”
Read previous RSNA News stories on cancer: