Deep Learning Model Analyzes Head CT to Predict sTBI Patient Outcomes
Model and clinical information can be used to predict 6-month outcomes after severe traumatic brain injury


Disability resulting from severe traumatic brain injury (sTBI) affects 55 million people globally each year, and 5 million people in the U.S.
In the 72 hours after a comatose sTBI patient is admitted to the hospital, physicians often make critical care decisions, including to continue or withdraw life-sustaining treatment, but most of the time they don’t have sufficient information and tools available to make reliable prognoses in clinical settings.
In response to this issue, a Radiology study examined the ability of AI to predict mortality or unfavorable outcomes of sTBI patients, based on the analysis of multi-modality data including head CT scans.
A Combined Human and Machine Effort
The study retrospectively analyzed two sTBI patient cohorts: one internal cohort of 537 patients from the University of Pittsburgh Medical Center (UPMC), and an external cohort of 220 patients from the Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) consortium. All patients were assessed for neurologic outcomes at three, six, and/or 12 months using the Glasgow Outcomes Scale.
Using admission head CT images and clinical data from a UPMC data set, the researchers trained a deep learning (DL) model to predict sTBI patient mortality and unfavourable outcomes at six months post-injury. The model was tested on the UPMC test data set and then the external TRACK-TBI data set. In order to evaluate the performance of the model, the researchers also compared it to other prognosis models, including the International Mission on Prognosis and Analysis of Clinical Trials in TBI (IMPACT) model and to the predictions made by neurosurgeons using imaging and clinical data.
Senior author Shandong Wu, PhD, associate professor in radiology, biomedical informatics, and bioengineering, and founding director at the University of Pittsburgh Center for Artificial Intelligence Innovation in Medical Imaging, emphasized that the goal of the research was to show how AI can augment clinical expert assessments to better serve sTBI patients.
“Our intention was to develop a multimodal model that incorporates AI analysis of CT images with clinical data to integrate all available information in that time-sensitive window after admission. We hope that a model such as this one can provide a more accurate prediction of a sTBI patient’s survival and recovery, which can better inform clinicians’ decisions and facilitate consulting with the patient’s family,” he said.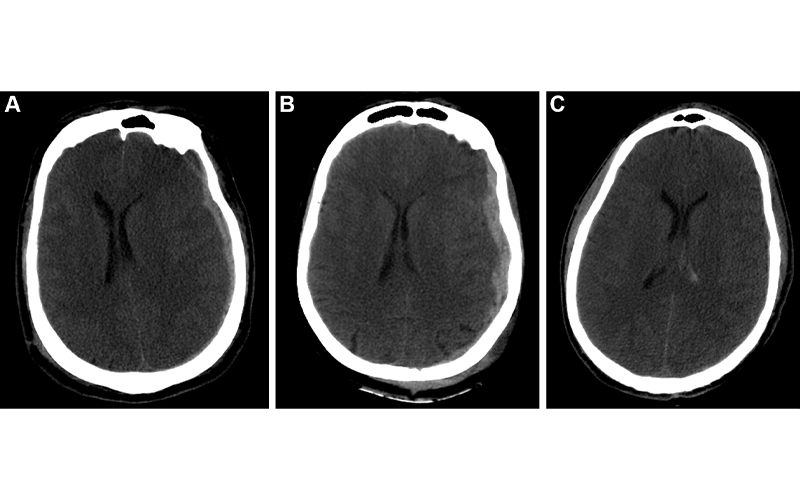
Example predictions by fusion model on University of Pittsburgh Medical Center patients. (A) Correct prediction in a 44-year-old man who was involved in an unrestrained motor vehicle collision. He underwent emergent decompressive hemicraniectomy (DHC), had bilateral lung injuries, and ultimately developed a pulmonary embolism with difficulty oxygenating on posttrauma day 6. His care was withdrawn, and he died. The model correctly predicted mortality. (B) Incorrect prediction in a 57-year-old woman who was in a motor vehicle collision and underwent DHC. The model predicted she would die, but she had a Glasgow Outcomes Scale of 3 at 2 years after trauma. She lived in a nursing home and was dependent on others for most daily living activities. (C) Incorrect prediction in a 28-year-old man who was in a motorcycle collision and had a minor head injury with intraventricular hemorrhage. Several weeks after trauma, he developed Klebsiella ventriculitis and pneumonia that led to an episode of severe hypotension. He subsequently developed malignant cerebral edema and died by brain death criteria. While the model predicted this patient would survive, this scenario highlights the difficulty of predicting outcomes based on information available in the emergency department, as events later in the patient’s course affect outcomes. https://doi.org/10.1148/radiol.212181 ©RSNA 2022
The Importance of External Validation and Heterogeneous Data Sets
The study results indicate that within the internal UPMC cohort, the fusion model, which combined both DL of CT images and clinical data, was the most accurate in predicting patient outcomes. It outperformed both the IMPACT model and neurosurgeons on predicting mortality and unfavourable outcomes. Within the external TRACK-TBI cohort, the performance of the fusion model dropped. Its performance was no different for predicting mortality, and worse for unfavourable outcomes.
For the researchers, this demonstrates the importance of external validation DL prediction models, as well as the necessity of training any AI prediction tool with a heterogeneous data set.
“The TRACK-TBI cohort was much more diverse, and some of the patient characteristics were statistically significantly different from the UPMC cohort; as a result, the fusion model didn’t perform as well as it did with the UPMC cohort, which was as expected,” Dr. Wu explained. The researchers are working on new AI techniques toward improving the model’s generalizability.
Implications for Use in Clinical Settings
Sven Haller, MD, neuroradiologist and medical director at Centre d’Imagerie Médicale de Cornavin in Switzerland, and visiting professor at the University of Uppsala, Sweden,
and Tiantan Hospital, China, highlighted in his commentary that the predictions of the neurosurgeons, which were at times not as accurate as other models, do not necessarily reflect their predictive expertise because the study did not accurately recreate a real-world setting for measuring their performance.
He did, however, point out the value of any prognostic tool that does not rely on a human operator—and therefore human inconsistency—used in conjunction with human insights.
“It's good to use a tool which is not potentially confounded by human emotions and biases, which impact performance. Ideally, it’s good to use both: the operator-independence of an automatic classifier, and the clinical experience by a human,” he said.
Dr. Haller also sees value in a quick AI prognostic tool that could decrease cost and increase efficiency in underfunded and overloaded health care systems.
“Where cost and resources are a concern, it would be possible to do a short clinical assessment followed by a short AI assessment,” he explained. “If they converge to either a good or bad outcome, physicians could confidently act on that information. Probably in the minority of cases, those assessments would not converge, leading to additional tests.”
Dr. Wu believes this study is encouraging and that AI is a potentially powerful tool for sTBI patient prognosis; however, he stressed that AI tools must be rigorously tested and validated before being used in any clinical setting.
“We learned to be cautious about the use of AI in prognosis,” he concluded. “In order for our model to be generalizable to other settings, it must be comprehensively evaluated further, including validation using more external data.”
For More Information
Access the Radiology study, “Outcome Prediction in Patients with Severe Traumatic Brain Injury Using Deep Learning from Head CT Scans."
Read RSNA's position statement on Traumatic Brain Injury (TBI) Imaging.
Read previous RSNA News stories on traumatic brain injury: