Deep Learning Helps Provide Accurate Kidney Volume Measurements
Measurements help evaluate disease progression and treatment direction


An AI model accurately performs total kidney volume measurements from MRI and reduces contouring time, potentially boosting treatment for people with a common type of kidney disease, according to research published in Radiology.
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disorder characterized by the formation of numerous cysts and scarring of the kidneys. It is the most common inherited disorder of the kidneys, affecting more than 12 million people worldwide. The disease progresses over time, with many patients experiencing kidney failure in their fifties and sixties.
Measurements of total kidney volume help clinicians evaluate ADPKD progression and determine eligibility for treatment with tolvaptan, a drug that slows disease progression.
“When patients’ kidneys increase in size, that has prognostic implications and correlates with worsening renal function and ultimately the need for dialysis,” said study lead author Akshay Goel, MD, diagnostic radiologist and associate clinical research scientist at Weill Cornell Medicine in New York City. “Therefore, tracking volume is important.”
Accurate Automated Segmentation Time Reduced Expert Contouring Time
Dr. Goel and his colleagues at Weill Cornell Medicine developed an open source deep learning model and deployed it to automatically segment kidneys using MRI for determining total kidney volume in patients with ADPKD.
Kidney segmentation with MRI directly correlates to total kidney volume, Dr. Goel explained, as each voxel maps directly to volume measurement.
“Thus, calculating the clinical metric of total kidney volume from the 3D segmentation, a computer vision metric, is straightforward and only requires the voxel volume and number of pixels within the segmentation mask,” he said.
Previously, operator exhaustion from the tedious contouring task may naturally have led to human error, particularly given the precision required at contour borders. In this study, the researchers used model-assisted annotation to modify the contouring task, with the aim of reducing operator fatigue and enabling radiologists to produce more accurate contours of kidneys in ADPKD.
The model was developed using 213 abdominal MRI studies in 129 patients with ADPKD.
Researchers randomly divided the patients into training, validation and test sets for model development. External validation in 20 patients from outside institutions and prospective validation in 53 patients demonstrated accurate automated segmentation for total kidney volume estimation of polycystic kidneys and reduced expert contouring time. Evaluation on the first 50% of prospective cases showed a 51% mean reduction in contouring time, from almost 29 minutes to only 12 minutes.
“The model performance can be characterized as excellent and robust, with minimal mistakes in the segmentation task on prospective studies at Cornell and exams taken from external institutions,” Dr. Goel said. “Quantitatively, volume estimates are nearly perfect, with an average difference of 2% to 3% from carefully labeled radiologist examples.”
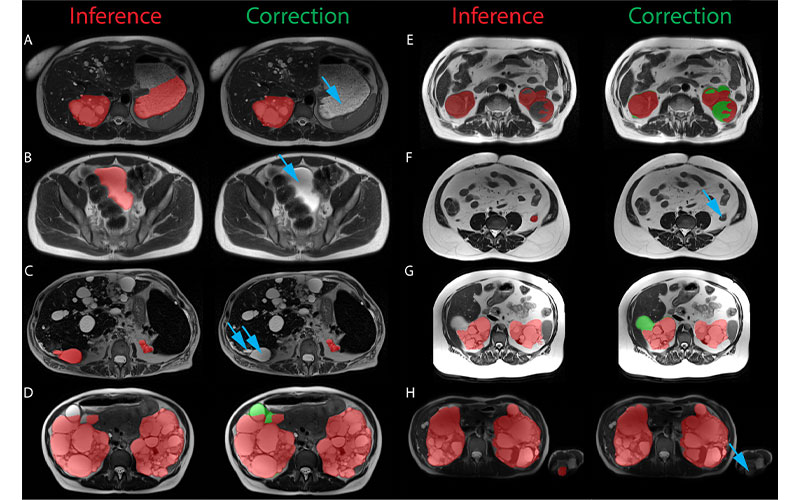
Examples of the most significant prospective model inference errors and the corresponding radiologist corrections. Inference label is red, radiologist additions are green, and radiologist subtractions are indicated by blue arrows. (A) Fluid-filled stomach partially labeled as left cystic kidney. (B) Urinary bladder labeled as cystic kidney. (C) Liver cyst labeled as kidney. (D) Renal cyst at liver border missed by inference. (E) Complex hemorrhagic left renal cyst incompletely labeled. (F) Collapsed descending colon labeled as left kidney. (G) Renal cyst at liver border missed by inference. (H) Left elbow medial epicondyle fat labeled as left kidney in a patient imaged with arms in the field of view. https://doi.org/10.1148/ryai.210205 © RSNA 2022
Model-Assisted Annotation Saves Substantial Radiologist Time
The findings are already having an impact on clinical practice, according to study senior author Martin R. Prince, MD, PhD, professor of radiology at Weill Cornell Medicine and Columbia University in New York City.
“Now that we have implemented this powerful deep learning technology into our clinical routine for adding accurate, reproducible organ volume measurements with a minimum of radiologist effort, we are seeing that referring physicians are utilizing these quantitative data enthusiastically,” Dr. Prince said. “Sometimes we are getting MRI studies ordered just to get these organ volumes. Patients are also focusing on these numbers and asking us to run the measurements on their outside or prior studies so they can get a better handle on their disease course.”
Drs. Prince and Goel and colleagues published a follow-up paper in Tomography in which the deep learning model reduced both the measurement variability and the radiologist time required to perform multiorgan segmentations in ADPKD.
For More Information
Access the Radiology study, “Artificial Intelligence Deployed Deep Learning Kidney Segmentation for Polycystic Kidney Disease MRI."
Access the open-source code repository at www.github.com/aksg87/adpkd-segmentation-pytorch.
Access the Tomography study at www.mdpi.com/journal/tomography.
Read previous RSNA News articles on kidney cancer: