Hybrid Magnetic Gold Nanoparticles Effective in Colorectal Cancer Treatment
Research highlights unique features of nanomaterials in diagnosing, imaging and treatment of colorectal cancer


Colorectal cancer, the fourth most common cancer diagnosed in the U.S. each year, is not only a ubiquitous cancer, it is a deadly one as well. Colorectal is the third most common cancer-related cause of death across the globe, according to the World Health Organization.
Upfront treatment of localized tumors usually involves a combination of surgical resection with or without adjuvant chemotherapy and/or radiotherapy. However, up to 25 percent of patients with colon cancer also present with synchronous metastases at diagnosis and a similar percentage of patients will eventually develop colorectal liver metastases.
Only about a quarter of patients with colorectal liver metastases are suitable candidates for surgical resection because of the size, number and location of their metastases.
So for a majority of patients, only non-surgical treatment options — such as chemoembolization, thermal ablation, 90Y radioembolization or external beam radiotherapy — are available. Many of these treatments, including thermal ablation, however, are typically effective only in tumors that are smaller than four or five centimeters in diameter. While in many cases radiotherapy can be utilized in larger tumors, that method has its own limitations in regard to liver and other normal tissue toxicities.
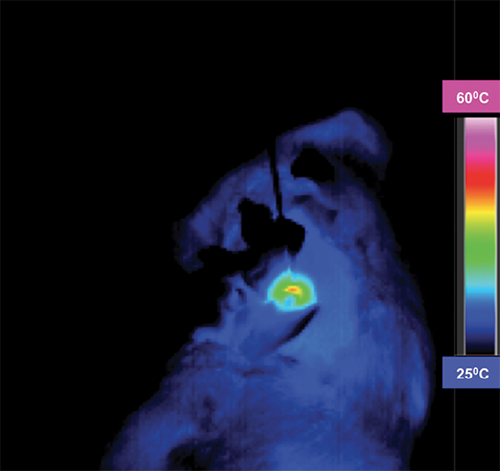
In recent Radiology research, lead author, Sarah B. White, MD, an associate professor of radiology, Medical College of Wisconsin, Milwaukee, and colleagues investigated whether bio-functionalized hybrid magnetic gold nanoparticles could be used to expand the size of ablation zones in order to treat larger colorectal cancer tumors with thermal ablation. These treatment algorithms include a nanomaterial system that permits MRI visualization and quantification and photothermal sensitization.
Dr. White has been particularly interested in developing new drug delivery systems that can be delivered site-selectively, which was the focus of her research project funded by a 2014-2016 Bracco Diagnostics Inc./RSNA Research Scholar Grant.
“In addition to or perhaps even instead of giving a patient systemic chemotherapy, we would use a nanoparticle as the carrier and deliver it directly to the tumor,” Dr. White said, noting that avoiding systemic treatment also will potentially prevent undesired toxicity to other organs.
“In this project we wanted to increase our ablative capability,” Dr. White said, pointing out that ablation can achieve local control for tumors that are three centimeters or less. “Beyond three centimeters, we are not as good as resection, but by increasing our ablative capability, we may in the future still be able to say that ablation can achieve durable local control."
Increasing the Size of Ablation Zones
Nanotechnology has developed over the last 20 years, said study author Dong-Hyun Kim, PhD, assistant professor, Department of Radiology, Feinberg School of Medicine, Northwestern University, Chicago, who operates a nanomedicine laboratory at the university to study multifunctional therapeutic nano-carriers for cancer treatment.
“Unique features of nanomaterials have been expected to provide new opportunities in early diagnosis, imaging and treatment of cancers. It should be advantageous, especially for tumor-localized cancer therapy,” Dr. Kim said.
In their study, Drs. White and Kim pointed out that sensitization with nanotechnology could potentially increase ablative capabilities without causing further toxicity and/or damage to a liver that may have already been exposed to hepatoxic chemotherapy.
Specifically, the researchers hypothesized that the use of anti-MG1 conjugated hybrid magnetic gold nanoparticles (HNPs) would act as a catalyst during photothermal ablation of colorectal liver metastases and increase the size ofablation zones.
“Conventionally, clinicians use laser to directly ablate the tumors,” Dr. Kim said. “In this case, we use the gold nanoparticles to increase the amount of heat generated.”
And since gold converts light energy — particularly laser light energy — into heat, it allows for the capability of a larger ablation zone, wherever the nanoparticles are deposited.
Getting the gold nanoparticles into the tumor was another challenge.
“We made nanoparticles with monoclonal antibodies that home in and bind to the tumor cell selectively,” Dr. White said. “They are endocytosed into the cell. When we shine the laser on the cell with the nanoparticles inside, the gold heats up and the cell dies.”
Using rat models, Drs. Kim, White and colleagues determined that these anti-MG1 HNPs are noncytotoxic and have a greater than 20 percent intra-tumoral accumulation compared with HNPs alone (nanoparticles that are not coated with the tumor specific monoclonal antibody). They also demonstrated that phototheramal ablation showed more than a two-fold enhancement in tumor response with the administration of anti-MGI HNPs vs. ablation alone.
“By increasing the ablation zone, we are no longer limited by three centimeters,” Dr. White said. “Maybe we can target a four-centimeter or five-centimeter tumor and get the same results.”
Clinical Applications Possible
In a Science to Practice article in the same issue of Radiology, Xiaoming Yang, MD, PhD, Department of Radiology, University of Washington School of Medicine, commented that the concept that targeted HNPs enhance the therapeutic effect of thermal ablation “presents an exciting strategy for complete removal of [colorectal liver metastases] by integrating two rapidly advancing scientific fields — in interventional radiology and nanotechnology.”
According to Dr. Yang, future application of this technique — if it proves successful in human subjects — could have further clinical implications.
For example, he suggested that the technique could include the co-loading or coating antitumor therapeutics in these targeted HNPs to promote therapeutic accumulation at tumor sites.
Dr. White and colleagues are now investigating other types of nanoparticles as well as alternate methods to deliver therapeutics and deposit them into tumors.
“In this study we delivered therapeutics via IV; however, there are problems with that method,” she said. “Administering nanoparticles site-selectively is on the horizon, as well as using the technique in other tumor models — not just colorectal liver metastases, but other cancers in the body.”

Web Extras
- Access the Radiology study, “Biofunctionalized Hybrid Magnetic Gold Nanoparticles as Catalysts for Photothermal Ablation of Colorectal Liver Metastases,” at RSNA.org/Radiology.