R&E-Funded Research Shows Potential for Improving Treatment Outcomes in Deadly Brain Cancer
The death of Senator John McCain — and before him, Senator Ted Kennedy — put a public face on glioblastoma, the most common malignant primary brain tumor diagnosed in adults and one of the most deadly.
“It’s a disease that afflicts people who are young and who are old, but most of the time it ends the same way,” said Daniel Wahl, MD, PhD, assistant professor in the Department of Radiation Oncology at the University of Michigan (UM), Ann Arbor. “Once it is diagnosed, it is essentially never cured. It is just intrinsically resistant to our current therapies.”
 With the aid of an RSNA Research & Education (R&E) Foundation Research Resident Grant, Dr. Wahl is working to break down this resistance, using novel metabolomics approaches to understand how cancer cells fend off radiation as they grow and divide. The work he began as a resident at UM — and continues in his faculty position — has the potential to change the storyline of this disease.
“This new avenue shows great promise, especially when combined with chemotherapy and radiation therapy, to improve the outcome of treatment,” said Theodore Lawrence, MD, PhD, Isadore Lampe Professor and chair of UM’s Department of Radiation Oncology, who served as Dr. Wahl’s supervisor on the project.
With the aid of an RSNA Research & Education (R&E) Foundation Research Resident Grant, Dr. Wahl is working to break down this resistance, using novel metabolomics approaches to understand how cancer cells fend off radiation as they grow and divide. The work he began as a resident at UM — and continues in his faculty position — has the potential to change the storyline of this disease.
“This new avenue shows great promise, especially when combined with chemotherapy and radiation therapy, to improve the outcome of treatment,” said Theodore Lawrence, MD, PhD, Isadore Lampe Professor and chair of UM’s Department of Radiation Oncology, who served as Dr. Wahl’s supervisor on the project.
Unlocking Metabolic Pathways to Cancer’s Growth
Treating glioblastoma is difficult for a number of reasons. Due to its location in the brain, surgeons cannot remove the tumor and leave a necessary margin of normal tissue surrounding the tumor; chemotherapy often cannot cross the blood-brain barrier. That leaves radiation, but glioblastoma has proven stubbornly resistant, Dr. Wahl said.
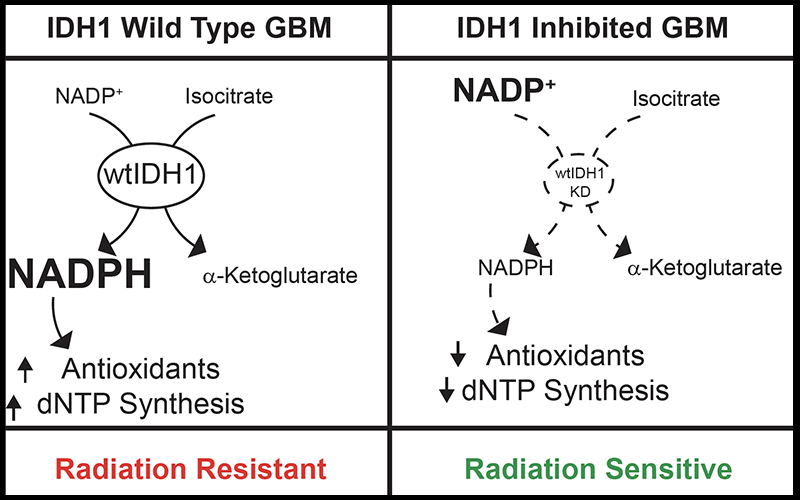
To understand why and to aid in future drug development, Dr. Wahl and his colleagues explored a key metabolic pathway called dihydronicotinamide-adenine dinucleotide phosphate (NADPH) production, or reductive biosynthesis. This process both allows cancer cells to grow and divide and produces deoxynucleotides and antioxidants that detoxify radiation. Seven or eight metabolic enzymes can produce NADPH. Dr. Wahl investigated multiple clinical data sets and found isocitrate dehydrogenase 1 (IDH1) to be the most upregulated NADPH-producing enzyme in glioblastoma, with levels nearly five times as high as in normal brain tissue.
In some types of tumors, IDH1 is mutated and other research groups are targeting this mutation as a therapeutic strategy. But Dr. Wahl took a different approach, looking at wild-type IDH1 in aggressive de novo glioblastomas without IDH1 mutations.
Once they identified IDH1, Dr. Wahl’s team genetically silenced wild-type IDH1 in glioblastoma models, both in vitro and in mice. Through funding from the RSNA grant, Dr. Wahl was able to forgo immortalized cell lines and use patient-derived glioblastoma models grown in clusters called neurospheres.
“Neurospheres resemble tumors more than just growing cells on the surface of plastic dishes,” Dr. Lawrence said. When IDH1 was knocked out, the models were much more sensitive to radiation. Assessing the models by mass spectrometry, Dr. Wahl found up to a 50 percent reduction in NADPH levels, as well as a 60 percent depletion of deoxynucleotides and a reduction in the antioxidant glutathione. As a final test, when those metabolites were reintroduced, cancerous cells were rescued, Dr. Wahl said.
“We can make a pretty safe connection that IDH1 makes NADPH, which makes antioxidants and deoxynucleotides, and those are the things that are important for resistance to radiation,” Dr. Wahl said.
Pinpointing Inhibition Strategies
Next, Dr. Wahl aimed to find compounds that could inhibit wild-type IDH1, beginning with those originally discarded as candidates for inhibiting IDH1 mutations. To do so, he developed an in vitroenzymatic assay.
Some compounds did not work while others did. Identifying which compounds to study further makes this assay an incredibly valuable tool, Dr. Wahl said.
“Without a way to tell in the lab whether something is targeting this enzyme or not, we are not able to push the research forward,” Dr. Wahl said. “The grant funding was completely necessary for that.”
Now that the grant has concluded and the results were published in Cancer Research in 2017, Dr. Wahl will continue to use his findings to test other compounds that may inhibit IDH1. He will also work to assess how the combination of inhibiting IDH1 and administering radiation affects cognition, inflammation and the overall health of normal brain tissue.
“We don’t want to make people feel any worse than they already do; quality of life is a huge issue for cancer patients,” he said. Besides its immediate impact, the research funded by the grant allowed Dr. Wahl to launch a career studying a disease in which outcomes are poor and new approaches are required. “It’s what drives me,” he said. “If we have enough creative ideas and ways to push this forward, something is going to work.”
Grants in Action:

Name:
Daniel Richard Wahl, MD, Phd
Grant Received:
2016-2017 Research Resident Grant
Study:
“Inhibiting Isocitrate Dehydrogenasel-Mediated Reductive Biosynthesis to Augment
Glioblastoma Therapy.”
Career Impact:
Published in the journal Cancer Research in 2017, the research served as a springboard for Dr. Wahl’s continued research at the University of Michigan. “The data from this grant served to support a successful grant application to the University of Michigan Center for the Discovery of New Medicines and as the foundation to begin my independent research career,” Dr. Wahl said.
Clinical Implications:
The results from this research show that inhibition of wild type IDH1, which is present in over 95 percent of glioblastomas, is an effective combination therapy with radiation and possibly chemotherapy. These results suggest that the development of therapeutic agents targeting wild type IDH1 could benefit patients with glioblastoma.