Radiologists Should Ready Their Practices For Automated US Breast Screening
Implementing AUS into workflow requires multidisciplinary training and communication


In concert with the RadioGraphics monograph, RSNA News shares the latest in breast cancer research, intervention and patient education.
In patients who can benefit from supplemental screening for breast cancer, automated ultrasound (AUS) is emerging as a solution to some major limitations encountered in clinical practice.
Radiologists at The University of Kansas Health System have outlined the potential benefits of AUS and offer practical guidance to help ensure that it’s implemented effectively.
In a RadioGraphics article, radiologists described the advantages AUS can offer over handheld US, overcoming operator dependence, enabling reliable image reproducibility, providing objective potential comparisons and potentially making audits less cumbersome. The authors warn, though, that the unique features of AUS can lead to high false-positive rates and longer interpretation times for the untrained eye.
“Familiarity with the common appearance of benign ultrasound findings, artifacts, technical challenges and unique AUS features is essential for fast, efficient and accurate interpretation,” said Ashley Huppe, MD, assistant professor of radiology and director of the breast imaging fellowship program at The University of Kansas Health System in Kansas City, KS. “While most radiologists are familiar with ultrasound, the automated technique has several unique features which radiologists should understand to improve diagnostic performance.”
Watch Drs. Huppe and Inciardi discuss the implementation of AUS and the importance of a multidisciplinary approach:
Looking For Cancer
Radiologists are aware of the challenges dense breast tissue poses on mammography and know that dense breast tissue is an independent risk factor for breast cancer. Co-lead author Marc Inciardi, MD, associate professor of breast imaging at the University of Kansas Health System, offers tips specific to interpreting findings on AUS.
“Multiple circumscribed masses are most commonly due to fibroadenomas, cysts or a combination of the two,” Dr. Inciardi said. “In our experience reviewing more than 80 cancers detected by AUS, we have not come across a cancer that mimics the appearance of a fibroadenoma— for example, a circumscribed parallel mass—or a cyst.”
“This statement may appear simplistic,” Dr. Inciardi added, “but cancers still look like cancers.”
He advised that any mass with suspicious features on AUS, such as non-parallel orientation, microlobulation or spiculation, should be considered suspicious and evaluated further with diagnostic US. In patients with fibrocystic breasts, Dr. Inciardi notes that radiologists should be familiar with a particular type of lesion: the papillary apocrine metaplasia or an acorn cyst.
“Appearing as a complex solid and cystic mass, this cyst is not uncommonly present in a background of diffuse fibrocystic disease,” he said, adding that radiologists should be familiar with its typical appearance in order to dismiss it as benign.
The authors recommend that in some instances, readers can use AUS features like skip artifact to their advantage.
“Try to confirm that the finding is present on two data sets,” Dr. Inciardi said. “We found that 85% of cancers are present on two data sets, if not in the peripheral breast. Assess its appearance primarily on the data set having the most compression, and recall the patient only if it is truly a mass with suspicious features. Use of the coronal plane in addition to the transverse plane can improve sensitivity, as a majority of cancers can be visualized in the coronal plane.”
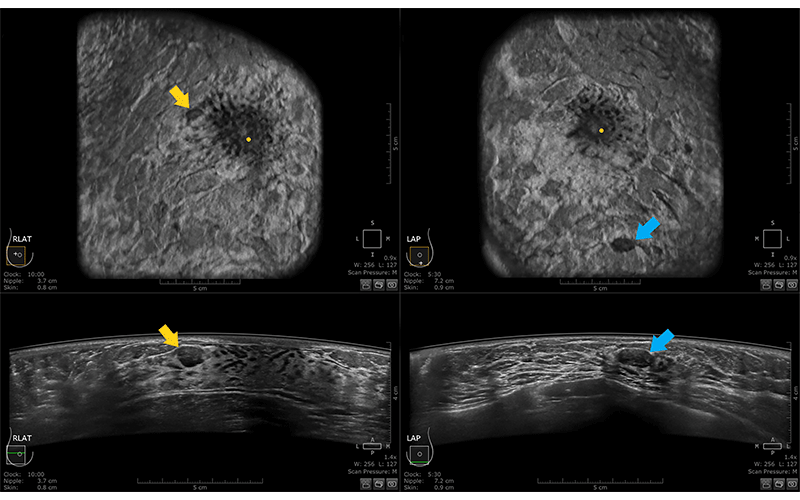
Multiple bilateral benign masses in a 44-year-old woman who presented for baseline screening AUS. Both coronal (top) and transverse (bottom) images from the LAT data set of the right breast (left) and the AP data set of the left breast (right) are shown. Oval, circumscribed, hypoechoic masses with parallel orientation are visualized in the upper outer right breast (yellow arrow) and the lower left breast (blue arrow). The patient had additional similar-appearing masses bilaterally, which fulfills criteria for a benign finding, or BI-RADS category 2. https://doi.org/10.1148/rg.230023 © RSNA 2023
Preparing to Implement AUS Into Workflow
The authors provided a comprehensive rundown of the unique features of AUS and how they stack up against handheld US. As for incorporating AUS into an imaging center’s workflow, they have solid advice as well.
At the University of Kansas, they’ve implemented a competency checklist for technologists who are new to AUS.
“We have found that it does improve technologist performance,” Dr. Inciardi said. “It gives the new technologist a clear set of steps to be completed. We recommend that every technologist, from the beginning, undergo assessment for at least 10 patients.”
Imaging practices should designate a lead radiologist and technologist to become “champions” of AUS, the authors advised. The lead radiologist should be available to answer questions from referring physicians and patients about how AUS screening fits in with existing technologies, to work with the breast center or radiology manager to set up a new workflow, and work with the lead technologist to ensure high-quality examinations.
“In some scenarios, this may require ‘lunch and learns’ or evening lectures, depending on the need,” Dr. Inciardi said. “The lead technologist is responsible for training—and retraining when necessary— other technologists.”
Radiologists should also give specific feedback to technologists to help improve their technique, reviewing the images together and discussing optimal positioning and compression.
Whatever the institution’s volume, Dr. Inciardi recommended that interpreting radiologists should aim to read at least five AUS exams each day, to keep their skills current and sharpen their performance after their initial training.
“Imagine starting a new career in radiology and just reading one or two mammograms per day; this would not be ideal,” he said.
If there’s a low volume of exams, he advises having them double read to maintain reading volume and skills.
“All abnormal AUS exams, after the diagnostic ultrasound is completed, should be reviewed by the radiologist who interpreted the initial AUS exam, even when the diagnostic exam was not performed by that radiologist,” he said.
Dr. Inciardi emphasized that this type of follow-up is critical to improve accuracy, and said that after the first year, any false-negative AUS exams should likewise be reviewed to see if there’s room for improvement in retrospect.
The University of Kansas team also finds it helpful to add a no-cost AUS exam at startup for every patient undergoing a US-guided biopsy, to build up a collection of at least 50 cases.
“One or two data sets over the area of interest can be done on the day of biopsy in about 10 minutes,” Dr. Inciardi said. “It will let radiologists and technologists quickly acquire a set of both benign and malignant findings for correlation between AUS and handheld ultrasound to improve interpretative skills.”
For More Information
Access the RadioGraphics paper, "Pearls and Pitfalls of Automated Breast Ultrasound (ABUS) interpretation."
Read previous RSNA News articles on breast imaging:
- Radiologists Play a Key Role in Assessing Radial Scars
- Understanding How Pregnancy and Breastfeeding Impact Breast Imaging and Diagnosis
- Enhancing the Evaluation of Breast Symptoms in the ED
- Closing the Breast Cancer Screening Gap for Uninsured Patients