RSNA Press Release
Molecular Breast Imaging May Benefit Women with Dense Breasts
OAK BROOK, Ill. – Screening women with dense breasts with both molecular breast imaging (MBI) and digital breast tomosynthesis (DBT) increased overall invasive cancer detection while modestly increasing the recall rate compared with screening only with DBT, according to a new study published today in Radiology, a journal of the Radiological Society of North America (RSNA).
"To our knowledge, this is the first multicenter, prospective evaluation of MBI as a supplement to DBT in women with dense breasts," said lead author Carrie B. Hruska, Ph.D., professor of medical physics at Mayo Clinic in Rochester, Minnesota.
An estimated 47% of women who undergo breast cancer screening have dense breasts, according to the Centers for Disease Control and Prevention. DBT is an advanced form of mammography that takes multiple X-ray images of the breast from different angles to create a 3D reconstruction of the breast, but it does not detect all breast cancers, especially in women with dense breasts.
MBI is one of several options available for supplemental breast screening for dense breasts, such as breast ultrasound, breast MRI and contrast-enhanced mammography. The Density MATTERS (Molecular Breast Imaging and Tomosynthesis to Eliminate the Reservoir) Trial was designed to assess the performance of screening MBI as a supplement to DBT in women with dense breasts.
For the trial, women with dense breasts from five sites were prospectively enrolled from 2017 to 2022 and underwent two annual screening rounds of DBT and MBI (prevalence screening at Year 1 and incidence screening at Year 2). One-year follow-up after each screening round was completed in September 2024.
Eligible participants included women aged 40–75 years who were asymptomatic and had dense breasts as visually assessed by a radiologist and reported on their last mammogram.
The study cohort included 2,978 participants (mean age 56.8 years). The women were mostly postmenopausal, and 82% had category C breast density. Approximately 80% of participants had no family history of breast cancer, and 98% had no personal history of breast cancer.
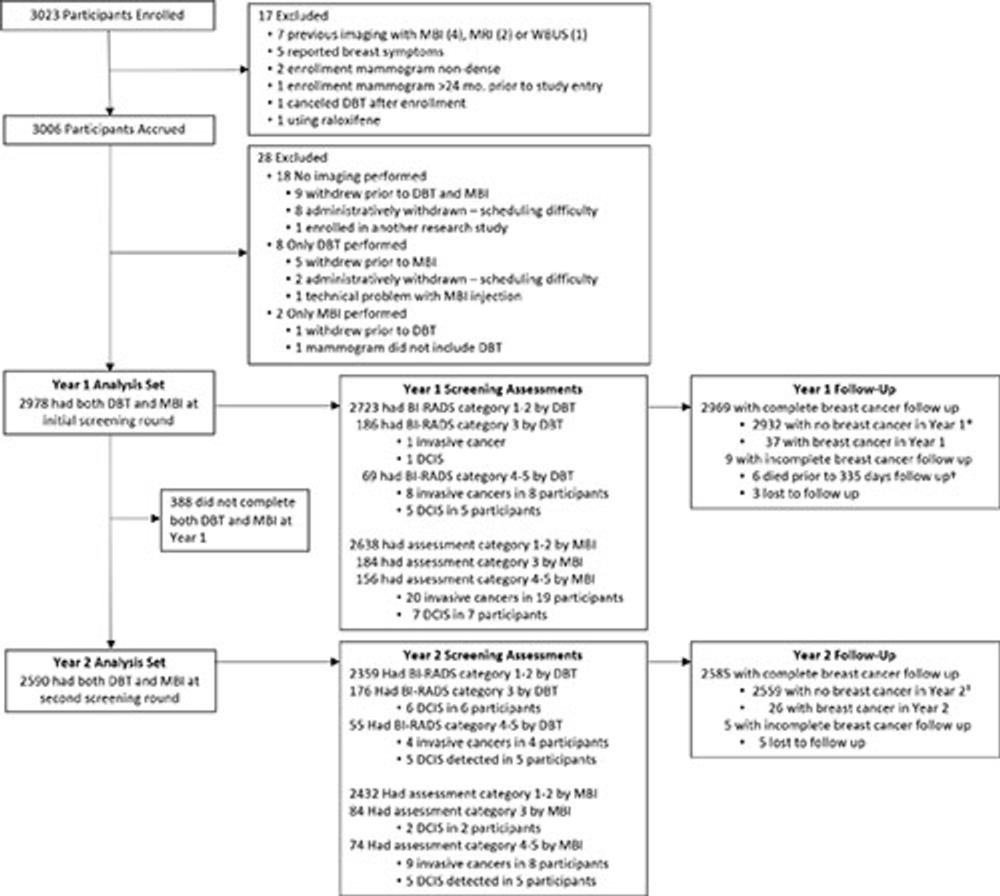
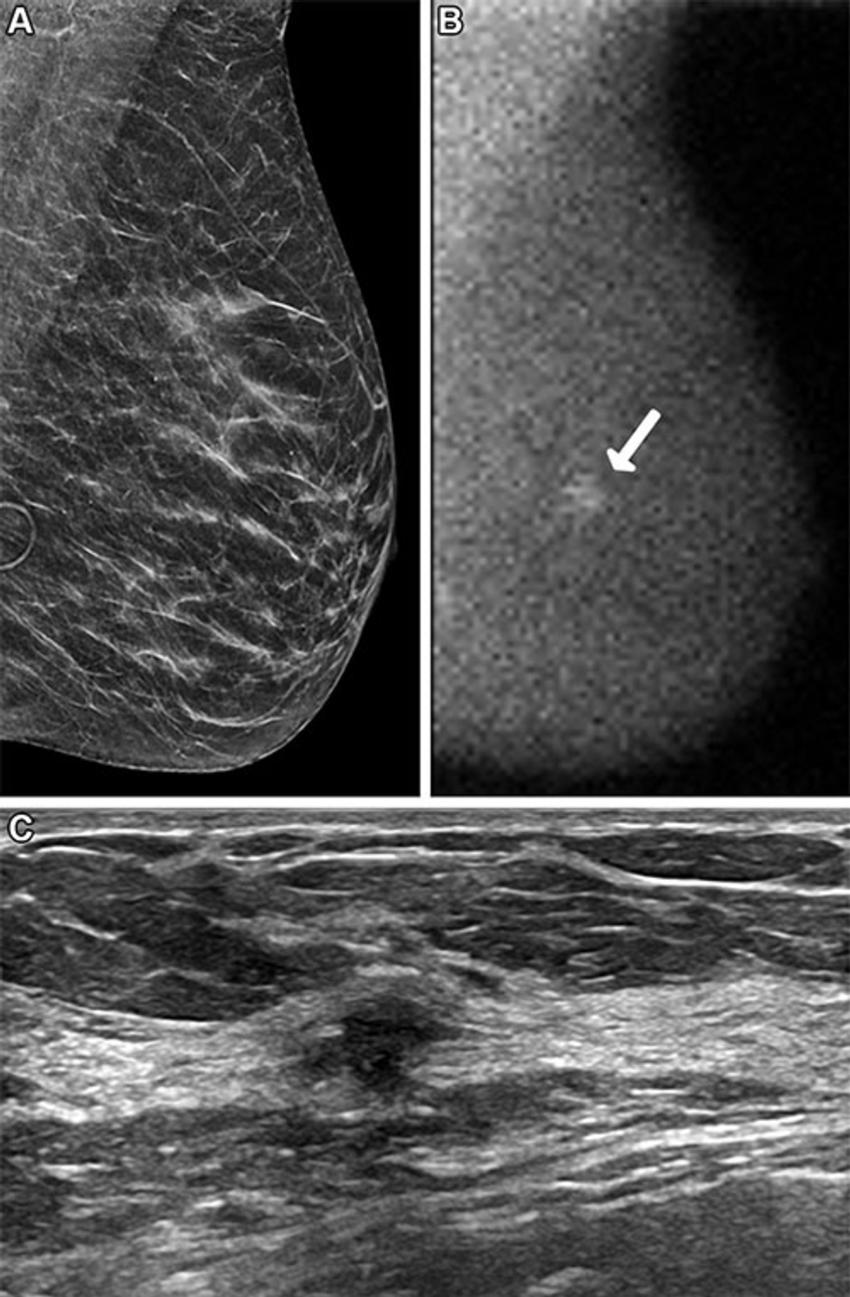
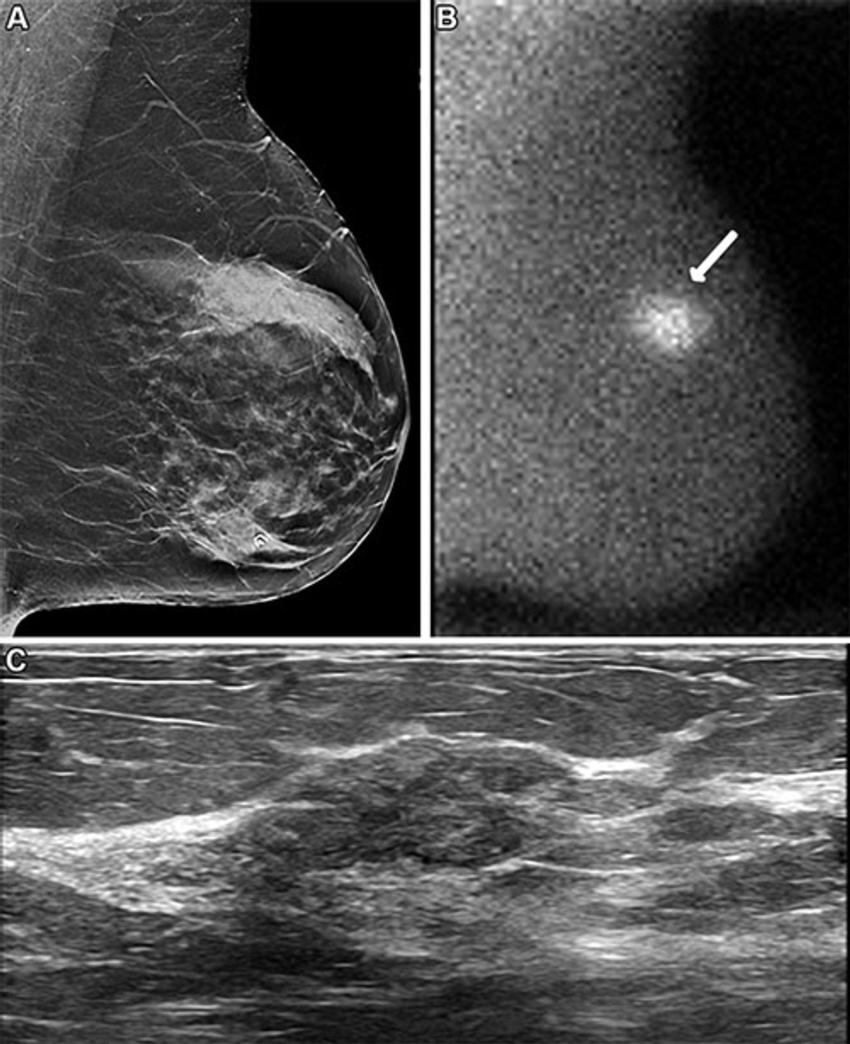
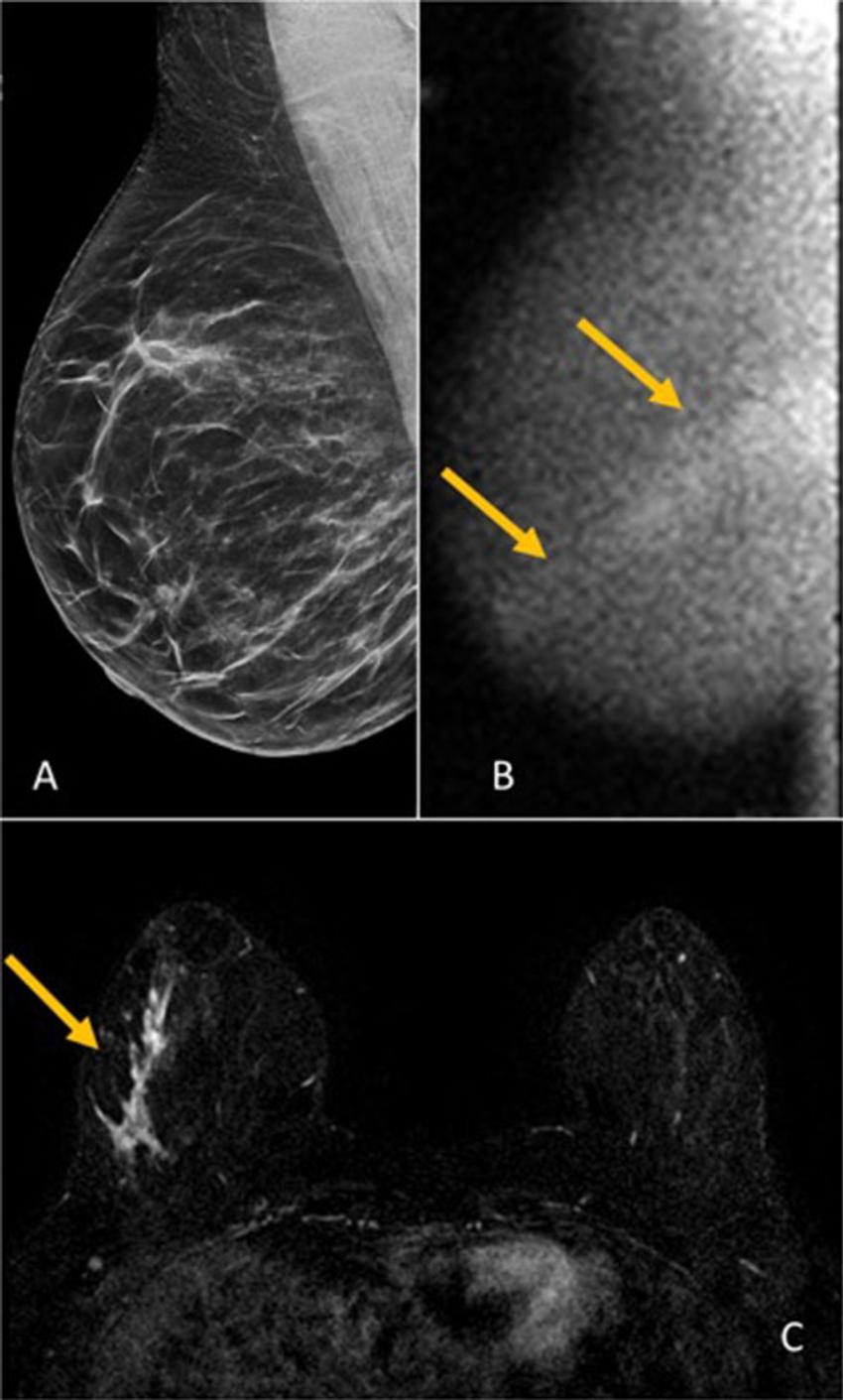
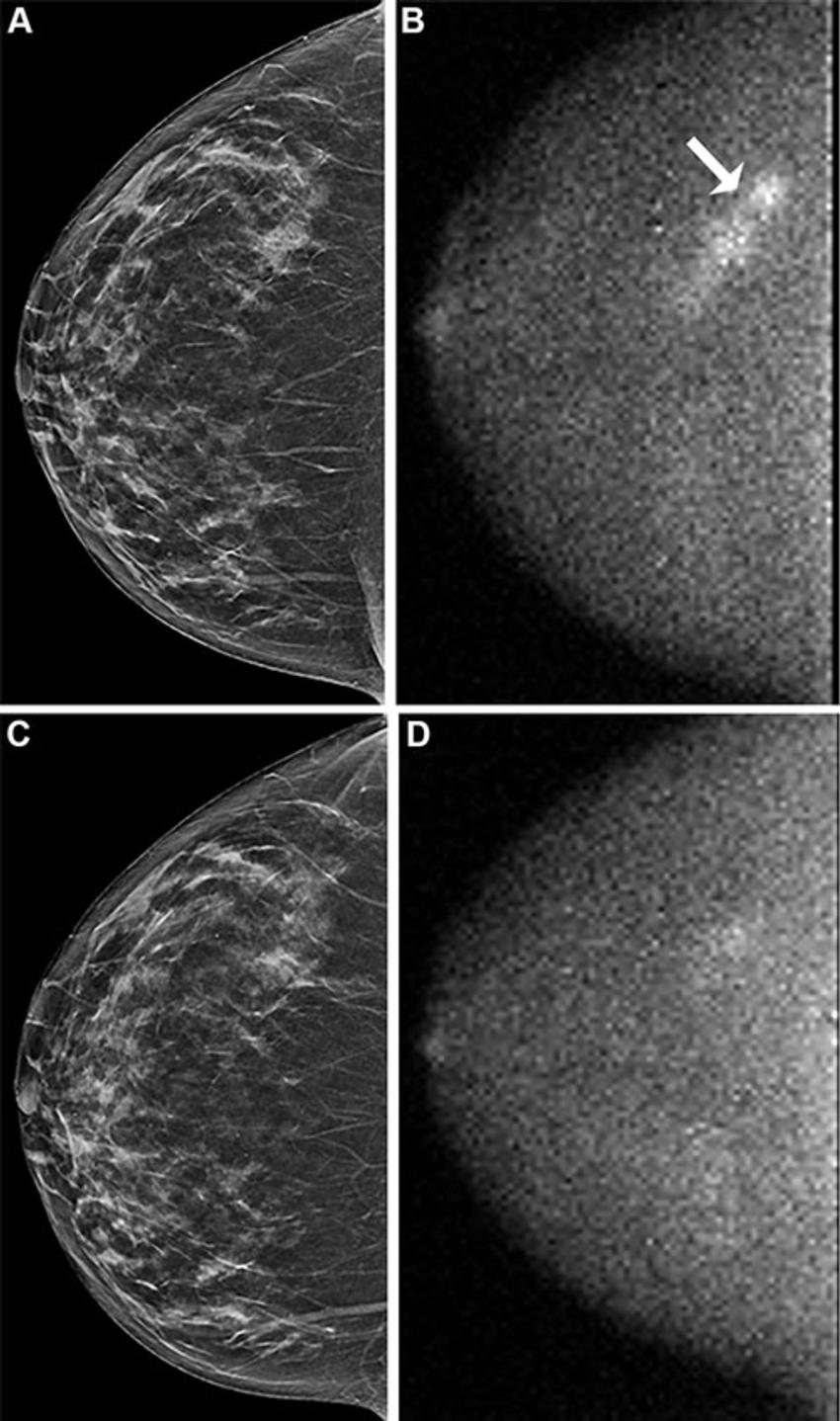
Across both screening rounds, 30 breast cancer lesions were detected in 29 participants by MBI only and not found with DBT. Most of these incremental breast cancers were invasive (22 of 30 or 71% of lesions). The median invasive lesion size was 0.9 cm. Among the participants with MBI-only detected breast cancer, 26 of 29 (90%) had node-negative cancers, and 6 of 29 (20%) had node-positive disease.
"MBI detected an additional 6.7 cancers per 1,000 screenings at Year 1 and an additional 3.5 cancers per 1,000 screenings at Year 2," Dr. Hruska said. "Among the incremental cancers detected only by MBI, 70% were found to be invasive. Additionally, 20% of those incremental cancers were node positive, suggesting that MBI can reveal mammographically occult, clinically important disease."
In the first screening round, 7 of 2,978 participants (2.4 per 1,000 screened) were diagnosed with node-positive cancers. DBT alone detected 4 of 7 (57%), and DBT plus MBI detected 7 of 7 (100%).
In Year 2, 6 of 2,590 participants (2.3 per 1,000 screened) had node-positive cancers. DBT alone detected 1 of 6 (16%) and DBT plus MBI detected 4 of 6 (67%). Neither modality detected 2 of the 6 node-positive cancers (33%).
"Someone who's having their routine annual screen every year should not be diagnosed with advanced breast cancer," Dr. Hruska said. "That's just unacceptable. With a supplemental screening every few years, we hope to find cancers earlier and see the diagnosis of advanced cancer go way down."
Dr. Hruska said one of the strengths of the Density MATTERS trial was the mix of academic medical centers and community hospitals involved. Participating centers also included MD Anderson Cancer Center, Henry Ford Health System, ProMedica Breast Care (regional practice in Ohio), and a Mayo Clinic Health System site in La Crosse, Wisconsin.
"The enrollment of 12% minority patients extends the generalizability of our findings," she said.
She said the trial results provide important data for healthcare institutions assessing the best modality for supplemental breast screening.
"I don't want to discourage anyone from getting a mammogram, because they absolutely should," Dr. Hruska said. "However, DBT doesn't find all cancers, and women need to understand its limitations and consider how supplemental screening can fill the gap."
According to Dr. Hruska, MBI is considered safe for routine screening, is well-tolerated by patients and is relatively inexpensive.
"MBI uses a well-established radiotracer that's been used in cardiac imaging for a really long time," she said. "It has fewer risks than other modalities and no contrast reactions. If a woman has a choice of modalities, it's important that she understands the benefits and risks of each and be involved in the decision-making."
"Molecular Breast Imaging and Digital Breast Tomosynthesis for Dense Breast Screening: The Density MATTERS Trial." Collaborating with Dr. Hruska were Katie N. Hunt, M.D., Nicholas B. Larson, Ph.D., Patricia A. Miller, M.D., Richard L. Ellis, M.D., Robin B. Shermis, M.D., Gaiane M. Rauch, M.D., Ph.D., Amy Lynn Conners, M.D., Jeannette Gasal Spilde, M.D., Dominic T. Semaan, M.D., Emily C. Siegal, M.D., Shannon N. Zingula, M.D., Sabala R. Mandava, M.D., Tamara S. Martin, M.D., Riffat K. Ahmed, M.D., Dana H. Whaley, M.D., Beatriz Adrada, M.D., Lacey R. Gray, C.N.M.T., Ramy A. Mehta, M.S., Rebecca J. Roll, Roberta E. Redfern, Ph.D., Michael O'Connor, Ph.D., and Deborah J. Rhodes, M.D.
Radiology is edited by co-interim editors Vicky Goh, M.B.B.Ch., King's College London, U.K., and Kathryn Fowler, M.D., University of California San Diego, California, and owned and published by the Radiological Society of North America, Inc. (https://pubs.rsna.org/journal/radiology) RSNA is an association of radiologists, radiation oncologists, medical physicists and related scientists promoting excellence in patient care and health care delivery through education, research and technologic innovation. The Society is based in Oak Brook, Illinois. (RSNA.org) For patient-friendly information on breast imaging, visit RadiologyInfo.org.Images (JPG, TIF):